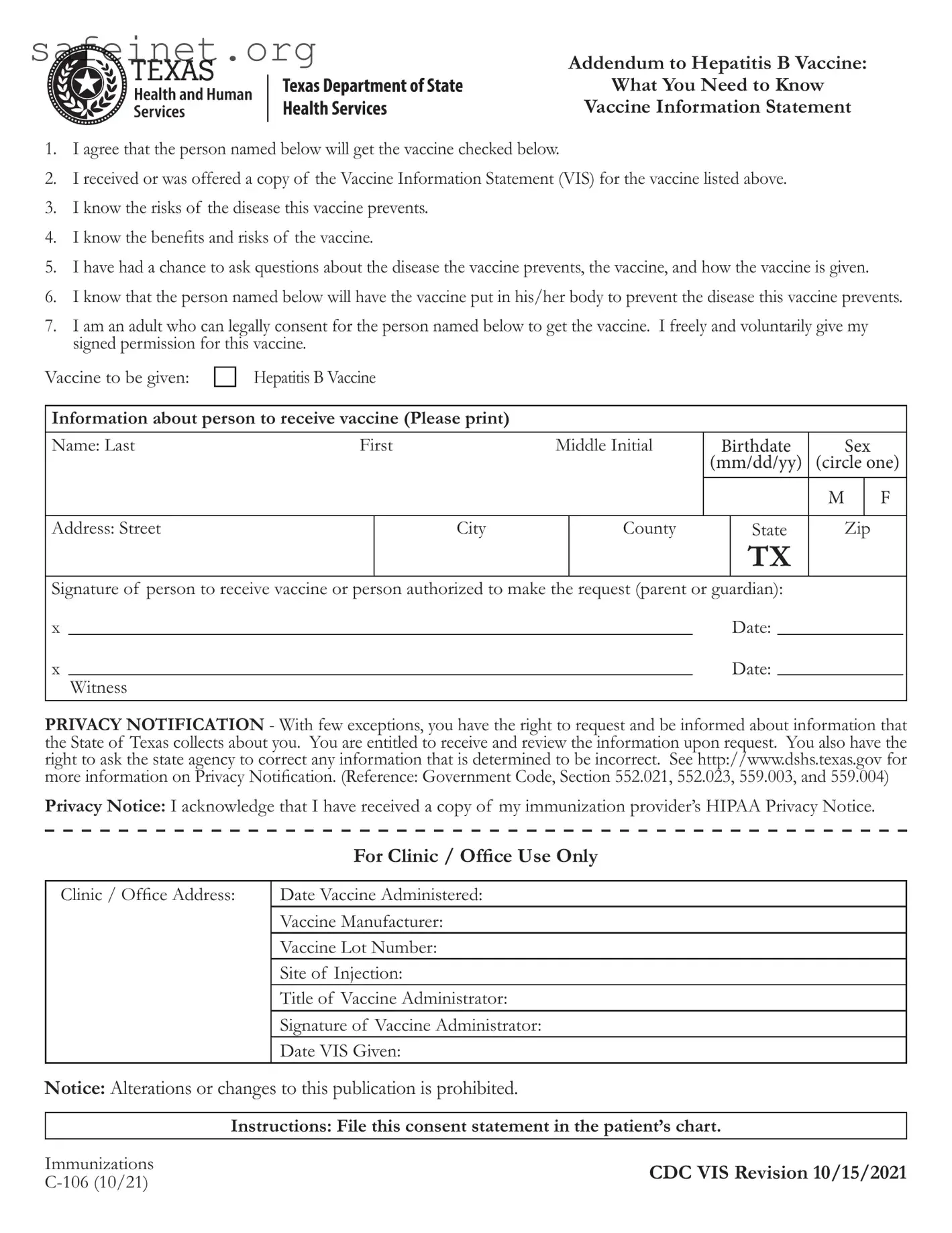
What is the purpose of the EC 106 form?
The EC 106 form serves as an addendum to the Hepatitis B Vaccine. It is designed to document the informed consent of individuals receiving the vaccine or the consent of a guardian if the recipient is a minor. The form ensures that the consent process includes acknowledgment of the risks and benefits associated with both the disease and the vaccine.
What information is required on the EC 106 form?
The EC 106 form requires specific information about the individual receiving the vaccine, including their full name, date of birth, sex, and address. Additionally, the form must contain the signature of the person receiving the vaccine or the authorized guardian, along with the date the consent was given.
What are the rights acknowledged in the EC 106 form?
This form acknowledges several rights related to privacy and informed consent. Recipients of the vaccine are informed that they have the right to request and receive information collected about them by the State of Texas. They also have the right to request corrections to any incorrect information. Furthermore, individuals are made aware of their right to receive a copy of the immunization provider’s HIPAA Privacy Notice.
Who can provide consent as indicated on the EC 106 form?
Only adults who are legally empowered to provide consent can sign the EC 106 form on behalf of the individual receiving the vaccine. This typically includes the recipient themselves if they are of age, or a parent or guardian if the recipient is a minor or otherwise unable to provide consent.
What should be done with the EC 106 form after it is completed?
After the EC 106 form is filled out and signed, it should be filed in the patient’s chart. This ensures proper documentation of consent for the administration of the Hepatitis B vaccine and maintains a clear record for medical and legal purposes.
Is it permissible to alter the EC 106 form?
No, alterations or changes to the EC 106 form are strictly prohibited. The integrity of the form must be maintained to ensure that the consent process is valid and legally acceptable. Any modifications could lead to complications regarding consent and documentation.