What is the purpose of the Contingency Plan For Vaccine Storage form?
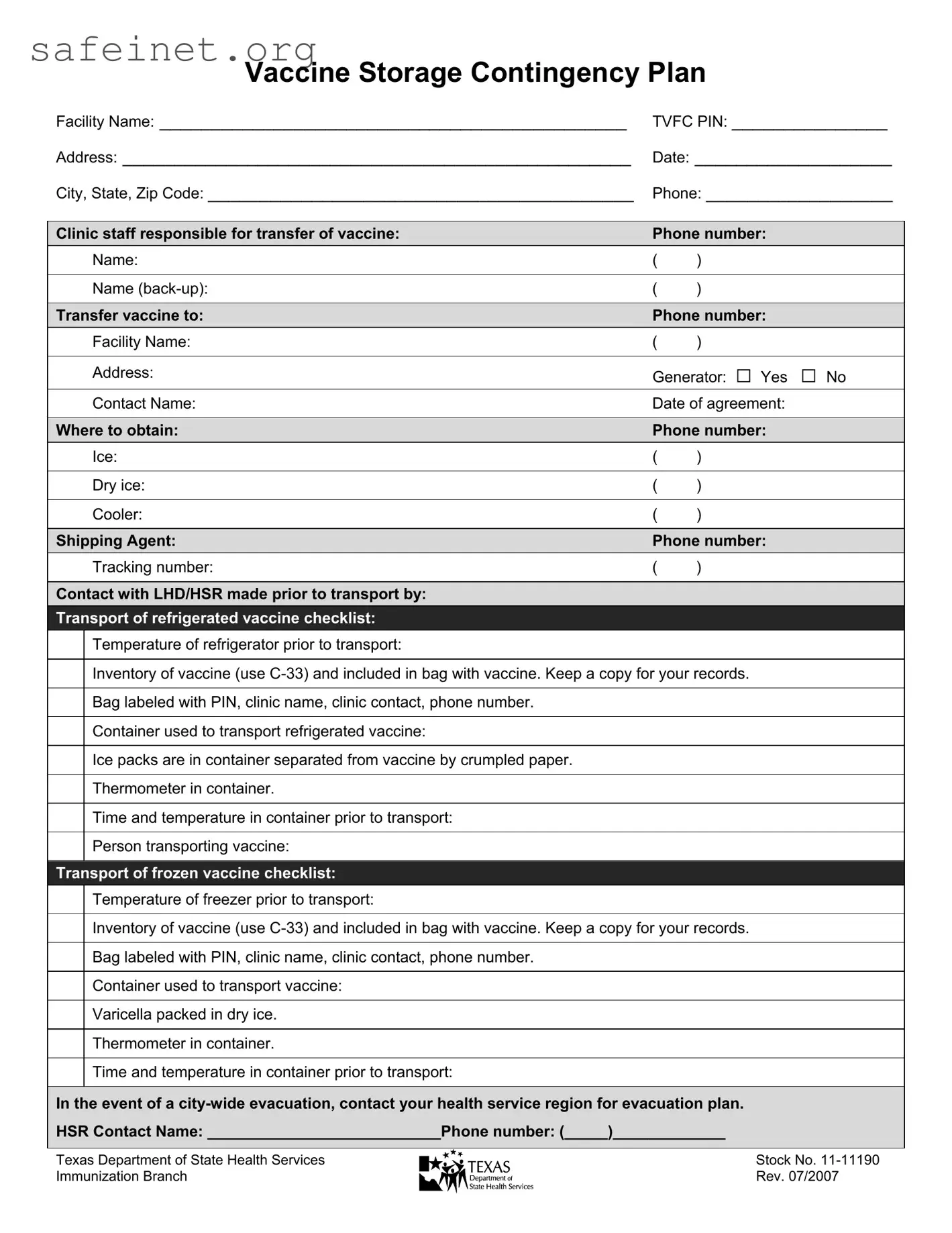
The Contingency Plan For Vaccine Storage form ensures that vaccines remain safe and effective during transport and in emergencies. In circumstances such as equipment failure or city-wide evacuations, this plan helps healthcare facilities establish protocols to preserve vaccine integrity.
What details are required for the facility on the form?
Facilities need to provide the facility name, Texas Vaccines for Children (TVFC) PIN, address, date, city, state, ZIP code, and a contact phone number. Accurate information helps maintain clear communication and coordination during vaccine transfer.
Who is responsible for the transfer of the vaccine?
The form requires identifying staff responsible for vaccine transfer, including a backup personnel. Recording their names and phone numbers ensures accountability and allows for easy communication should questions arise during the transfer process.
How should vaccines be transported, based on the form?
Vaccines must be transported in appropriate containers, using ice packs for refrigerated vaccines and dry ice for frozen vaccines. Staff should label all bags with the PIN, clinic name, contact details, and ensure that the contents remain at the required temperature throughout the transport process.
What preparatory checks need to be completed prior to transport?
Before transport, it is essential to check the temperature of the refrigerator or freezer. Additionally, an inventory of the vaccines should be recorded using the C-33 form, which should accompany the vaccine during transport. This documentation helps track what has been moved and ensures that the correct conditions are maintained.
What equipment is necessary for transportation?
Each transport must include a thermometer, ice packs for refrigerated vaccines, or dry ice for frozen vaccines. The form highlights that ice packs should be separated from the vaccines with crumpled paper to prevent direct contact that could affect temperature stability.
How should facilities prepare for a city-wide evacuation?
In a city-wide evacuation, facilities must contact their health service region (HSR) to follow the established evacuation plan. The form provides a space to document the HSR contact name and phone number, which is essential for obtaining direction during an emergency.
What should staff do if they need to make an agreement for vaccine transfer?
When transferring vaccines, the form has a section to document the name of the facility receiving the vaccines, the contact details, and the date of the agreement. Establishing this written agreement ensures that all parties are informed and prepared for the transfer, helping to prevent miscommunication.